New vaccine launched to help in the fight against calf pneumonia
2018 marks the final phase of a five-year AMR strategy (antimicrobial resistance) project which the farming industry, vets and policy makers have been part of with the aim of looking at how to best meet agreed AMR commitments. A fantastic start was made with a 27% reduction in antibiotic use in farm animals recorded in the two years to October 2017. Following this, RUMA (Responsible Use of Medicines in Agriculture Alliance) sector-specific targets, which were published in late 2017 gave clear areas of focus for farmers, vets and advisors.
For the dairy sector, calf rearing was identified as a ‘hot spot’ of treatment and a stage in the animal’s life where antibiotics could be used more appropriately and vaccination more widely integrated into herd management protocols.
Similarly, the calf stage for the beef sector was also singled out as another ‘hot spot’ with bovine respiratory disease (BRD) an area to focus on.
“There is also an emphasis on calves from dairy herds, where mixing animals from different sources can create a peak in disease pressure,” explains Matt Yarnall of Boehringer Ingelheim. “One of the main things farmers could do is to measure what is being used on-farm and monitor it on an ongoing basis. Many vets are now offering to undertake a ‘medicine audit’ and, with that information, you will know when progress is being made and areas to concentrate on. The majority of farms have the potential to use antibiotics more prudently somewhere.
“The levels of BRD vaccine being used each year is growing but, as a management tool, vaccination remains quite low down the list of actions, according to the 2017 Calfmatters survey,” remarks Matt Yarnall. See Graph 1.
Graph 1: shows farmers’ responses to being asked how they control pneumonia on their farms (Calfmatters survey 2017)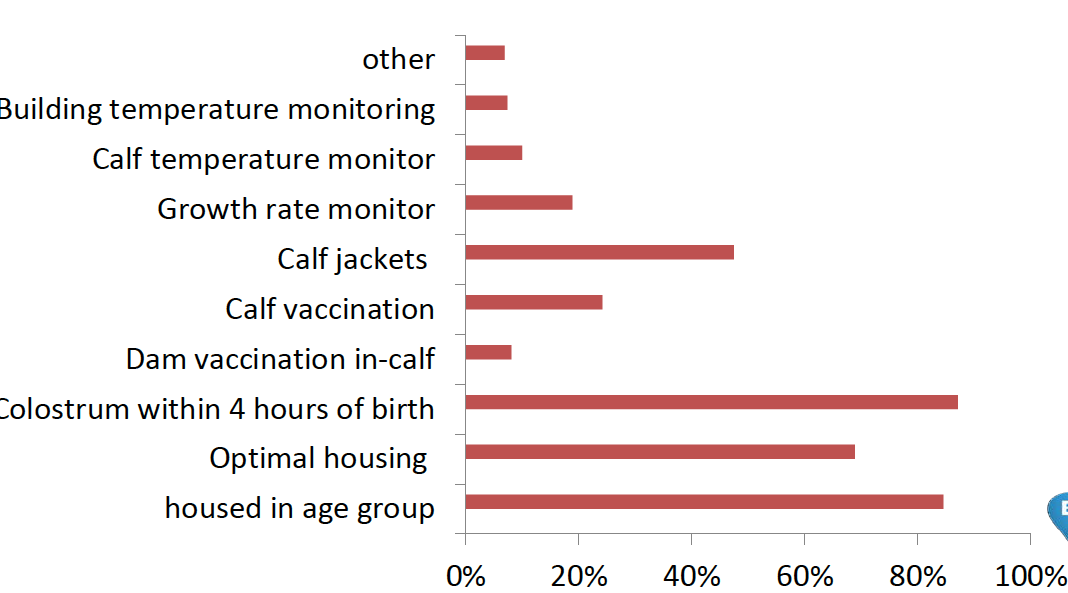
Offering farmers more choice and the opportunity to protect very young calves, Bovalto® Respi Intranasal has been launched by Boehringer Ingelheim - an intranasal vaccine for calves that can be used from 10 days of age.
Bovalto Respi Intranasal protects against the two main respiratory viruses – RSV and PI3, and during its development it was tested against the strains that are causing disease in stock currently1.
Being an intranasal vaccine, it triggers mucosal immunity in the nasal passages and throat area. This is an extensive immune system present in cattle, so priming it with a vaccine such as this delivers fast, effective, predictable protection.
Bovalto Respi Intranasal is delivered as a single shot, from 10 days of age and provides immunity for 12 weeks, from 10 days after vaccination. It has also been shown to the effective in the face of MDA (maternally derived antibodies).
In order to assist with effective administration, a dedicated administration pack for use has been developed. This includes the Bovalto Respisafe applicator and nozzle which fits against the calf’s nostril, along with a vaccinator.
“The aim is to make sure that vaccination is a comfortable process for both farmer and calf, as well as addressing concerns on the administration of vaccine to the appropriate area in the nasal passages,” adds Mr Yarnall.
Correct administration of the vaccine will ensure that the spray is delivered in the most efficient manner to do its job. If the droplet size is too big, then it can run out of the nose, and if too small then this is carried into the back of the throat and is wasted.
“Time and money is spent vaccinating calves and so the process needs to be effective to give optimal protection,” concludes Mr Yarnall. “If adding to the Bovalto range encourages more farmers to vaccinate against pneumonia so improving calf heath and lowering antibiotic use, that can only be a step in the right direction.”
Bovalto Respi Intranasal joins Bovalto® Respi 3 and Bovalto® Respi 4 in Boehringer’s BRD vaccine range. Bovalto Respi 3 and Respi 4 offer a proven six months of immunity against the bacterium Mannheimia haemolytica* A1 and the important viral causes of respiratory disease, Parainfluenza 3 Virus and Respiratory Syncytial Virus, with Bovalto Respi 4 also offering 6 months’ immunity against Bovine Viral Diarrhoea Virus1.

ENDS
- Philippe-Reversat et al. (2017) Acta vet. BRNO 86: 325-332
Bovalto Respi Intranasal, nasal spray, lyophilisate and solvent for suspension contains Bovine parainfluenza 3 virus (PI3V), modified live virus, strain Bio 23/A 105.0 – 107.5TCID50and Bovine respiratory syncytial virus (BRSV), modified live virus, strain Bio 24/A 104.0 – 106.0 TCID50. For the active immunisation of calves from the age of 10 days against bovine respiratory syncytial virus (BRSV) and bovine parainfluenza 3 virus (PI3V), to reduce the quantity and duration of nasal excretion of both viruses. Bovalto®Respi 3 Suspension for Injection and Bovalto®Respi 4 Suspension for Injection contain inactivated bovine respiratory syncytial virus, strain BIO-24, inactivated bovine parainfluenza 3 virus, strain BIO-23 and inactivated Mannheimia haemolytica, serotype A1 strain DSM 5283. Bovalto Respi 4 also contains inactivated bovine viral diarrhoea virus, strain BIO-25. UK: POM-V. For information about side effects, precautions, warnings and contraindications please refer to the product packaging and package leaflet. Further information available in the SPC or from Merial Animal Health Ltd, RG12 8YS, UK. UK Tel: 01344 746960 (sales) or 01344 746957 (technical). Bovalto and the steerhead logo are registered trademarks of Merial. ©2018 Merial Animal Health Ltd. All rights reserved. Merial is now part of the Boehringer Ingelheim Group of Companies. Date of preparation: Jul 2018. AHD11198. Use Medicines Responsibly.
